The two most common genotypes causing sickle cell disease (SCD) are homozygous bsbs producing hemoglobin S (HbSS), and compound heterozygous bsbc producing HbS and HbC (HbSC). HbS polymerizes in both, when deoxygenated. Patients with HbSC have distinct hematologic characteristics such as higher Hb levels but lower fetal hemoglobin (HbF); increased corpuscular Hb concentration, due to pronounced red blood cell (RBC) K+ loss and dehydration, contributes to HbS polymerization. While hydroxyurea (HU) as a HbF-inducing agent clearly improves HbSS phenotype, targeting of other pathogenetic mechanisms in SCD may offer additional benefit in both HbSS and HbSC patients.
The RBC metabolism depends on anaerobic glycolysis for ATP production. Key RBC functions are intricately linked to metabolism: hemoglobin-oxygen (Hb-O2) affinity is modulated by 2,3-diphosphoglycerate (2,3-DPG), RBC membrane health depends on adequate synthesis of ATP, and heme-iron is maintained in the functional reduced state by the synthesis of antioxidants, such as GSH, supplying NADH to methemoglobin reductase. Erythrocyte pyruvate kinase (PKR) performs a rate limiting step in RBC glycolysis, converting phosphoenolpyruvate to pyruvate and generating ATP. Insufficient PKR activity due to any of a variety of causes, including excessive energy demand, can alter the abundance of metabolic products and intermediates, including ATP and 2,3-DPG, adversely affecting RBC function.
We assessed baseline 2,3-DPG and ATP levels in whole blood of patients with HbSS (N=18) by LC/MS analysis. As well-reported, hemoglobin (Hb) levels were significantly decreased in patients with HbSS compared to healthy volunteers (9.68 ± 0.32 g/dL vs 14.88 ± 0.27 g/dL, p<0.0001). After normalizing to the decreased Hb levels, 2,3-DPG levels were significantly increased (4842.78 ± 182.98 vs 4327.73 ± 126.60 µg/g Hb, p<0.05) and ATP levels were significantly decreased (1621.27 ± 133.02 vs 1995.37 ± 60.80 µg/g Hb, p<0.05) in whole blood specimens of patients with HbSS compared to healthy volunteers. FT-4202 is a potent, selective, and orally bioavailable allosteric activator of PKR which is in Phase 1 clinical trial for the treatment of SCD (NCT03815695). We have previously shown that FT-4202 decreases 2,3-DPG within 24 hr of single dose and increases ATP after 14 days of multiple dosing in healthy volunteers (Blood, 2019, 134, Supplement_1:616). Here, we hypothesized that, by reducing 2,3-DPG and increasing ATP, FT-4202 may improve oxygen affinity and membrane health in both HbSS and HbSC RBCs irrespective of hydroxyurea use and HbF expression.
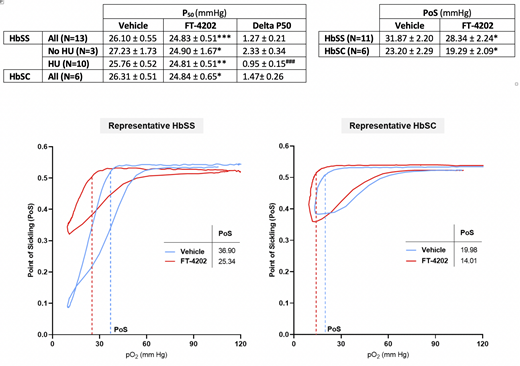
Whole blood samples in K2EDTA were collected from donors with confirmed HbSS or HbSC disease in accordance with an approved IRB protocol in Cincinnati Children's Hospital. % HbF and % F-cells were measured for each sample. Packed RBCs were washed, resuspended to 20% v/v (20% hematocrit), and then incubated for 4 hr at 37oC with 20 μM FT-4202 or equivalent volume of vehicle control DMSO. P50 representing the partial pressure of oxygen at which Hb is 50% saturated with oxygen, was determined using non-linear regression analysis of hemoglobin-oxygen equilibrium curves obtained on a HEMOX Analyzer (TCS Scientific). RBC deformability as a function of oxygen pressure, or Oxygenscan, was measured using Lorrca® (RR Mechatronics) (Rab et al., 2019, Am J Hematol). Point of Sickling (PoS) was calculated as the pO2 at which RBC deformability is decreased by 5% of maximum value during deoxygenation. The P50 and PoS values in FT-4202 versus DMSO-treated HbSC or HbSS RBCs from patients treated with or without HU were analyzed by paired Wilcoxon test. FT-4202 treatment significantly decreased P50 in both HU-treated and not HU-treated HbSS RBCs, as well as in HbSC RBCs, as detailed in Table below showing mean ± se (*p<0.05, **p<0.002, ***p<0.0005 for FT-4202 versus vehicle). The decrease in P50 was significantly higher in not HU- versus HU-treated HbSS RBCs (###p<0.001 by two-way ANOVA) likely due to the increased HbF with baseline decreased P50 in HU-treated RBCs (23.6 ± 2.3 %HbF) versus non-HU treated RBCs (10 ± 1.9 %HbF). PoS was decreased after FT-4202 treatment both in HbSS and HbSC RBCs (p<0.05).
These data suggest that increasing flux through the glycolytic pathway by FT-4202 treatment may offer dual beneficial effects of increased Hb-O2 affinity and membrane flexibility in HbSC as well as in HbSS RBCs irrespective of HU use.
Fulzele:FORMA Therapeutics, Inc: Current Employment, Other: Shareholder of Forma Therapeutics. Guichard:FORMA Therapeutics, Inc: Current Employment, Other: Shareholder of Forma Therapeutics; AstraZeneca: Other: Shareholder. Kalfa:Agios Pharmaceuticals, Inc: Consultancy, Research Funding; Forma Therapeutics, Inc: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal